valence electrons chart|how to calculate valence electrons : Tagatay Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called . 48 talking about this. Slotaholic is a gaming channel created by slot machine enthusiast Josh Duffy.
PH0 · which element will have 1 valence electron
PH1 · valence electrons table of elements
PH2 · valence electron periodic table chart
PH3 · valence electron configuration chart
PH4 · periodic table with valence electrons pdf
PH5 · list of valence electrons for each element
PH6 · how to count valence electrons periodic table
PH7 · how to calculate valence electrons
PH8 · Iba pa
From the creators of Canada’s leading sports media app, theScore, comes the sportsbook and casino app that you’ve been waiting for. theScore Bet is an award-winning sportsbook that is uniquely integrated with theScore, allowing you to seamlessly navigate between the betting app and real-time news, stats and scores.
valence electrons chart*******Mar 23, 2023 Find the maximum and most common valences of the elements in a table .Learn how to determine the number of valence electrons for main group elements using the periodic table. See patterns, examples, and tips for identifying valence electrons on .
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine the element's chemical prope.Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s 1 s sublevel are called .
Explain the relationship between the chemical behavior of families in the periodic table and their valence electrons. Identify elements that will have the most similar properties to a given element. The . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical .Learn how valence electrons determine chemical properties and bonding of elements. Find the number of valence electrons for each element in the periodic table and see examples of valence electron configurations.
valence electrons chart how to calculate valence electrons The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 4.4.2 4.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr. Valence Electrons Chart of Elements (With Periodic table) May 27, 2023 by Jay Rana. Periodic table with valence electrons is shown in the above image. Points to remember: Valence electrons are the .
The oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. A lithium atom has one outer shell electron. It has a .how to calculate valence electronsElectrons that are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency (or valence) of an atom. . The general oxidation state of the elements of the periodic table is illustrated in the chart provided below. Valency of First 30 Elements. The valency of the first 30 .
First Ionisation Energy1312 kJ/mol. Atomic Radius 53 pm. Covalent Radius 37 pm. Thermal Conductivity0.1805 W/ (m K) Specific Heat 14300 J/ (kg K) Heat Fusion 0.558 kJ/mol. Heat Evaporation0.452 kJ/mol. Molar Volume22.4135 cm3/mol. Lattice Constant 470 pm.
The number of electrons in the outermost shell of a particular atom determines its reactivity, or tendency to form chemical bonds with other atoms. This outermost shell is known as the valence shell, and the electrons found in it are called valence electrons. In general, atoms are most stable, least reactive, when their outermost electron shell . Figure 5.2.1 5.2. 1: (a) The radius of an atom is defined as one-half the distance between the nuclei in a molecule consisting of two identical atoms joined by a covalent bond. The atomic radius for the halogens increases down the group as n increases. (b) Covalent radii of the elements are shown to scale. Solution. Element A is located in Period 2, the 5th position in 2p-block.Before the electrons are placed in 2p subshell, the 2s subshell must be filled first. This means that A has two valence electrons in 2s (2s 2) and five valence electrons in 2p (2p 5).Answer: 2s 2 2p 5. It has 2 + 5 = 7 valence electrons.. Element B is located in Period 3, the 2nd .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .1.3: Valence electrons and open valences. A valence electron is an electron that is associated with an atom, and that can participate in the formation of a chemical bond; in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The presence of valence electrons can determine the element's .
10.6: Valence Electrons is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The arrangement of electrons in atoms is responsible for the shape of the periodic table. Valence electrons are those electrons in the highest energy level of an atom. Thus, the number of valence .. Note 1: If you want the valence electrons of all the 118 elements, then visit this article: Valence electrons chart for ALL ELEMENTS (Where I have shown the valence electrons using images). Note 2: If you want a periodic table with valence electrons labeled on it, then visit this article: Periodic table with Valence electrons . And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system . The stronger pull (higher effective nuclear charge) experienced by electrons on the right side of the periodic table draws them closer to the nucleus, making the covalent radii smaller. Figure 8.4.2 8.4. 2: Within each period, the trend in atomic radius decreases as Z increases; for example, from K to Kr.
Hence it has 2 valence electrons. Valence electrons can also be determined as the electrons present in the shell with highest principal quantum number (n). For example, The electron configuration of magnesium is 1s 2 2s 2 2p 6 3s 2. Here, you can see that the highest principal quantum number is 3, and the total electrons in this .
Characteristics of Valence Electron. Electrons are involved in the chemical bonding and reactions of the atom. It is said to occupy orbitals in an atom. The number of valence electrons of an atom can be obtained from the periodic table because it is equal to the group number of the atom. Atoms are most stable if they have a filled valence shell . This printable periodic table contains the atomic number, element symbol, element name, atomic weights and most common valence charges. Todd Helmenstine. This table also contains the element number, element symbol, element name, and atomic weights of each element. This periodic table in PDF format can be downloaded for printing.

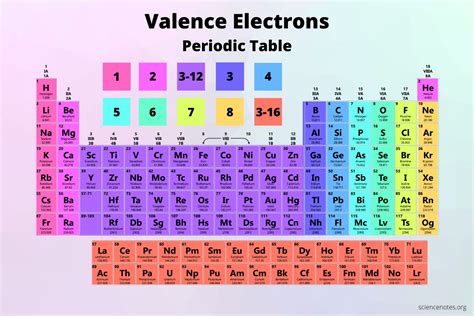
Valence Electrons Periodic Table Chart. The table below depicts the number of valence electrons in the different groups of the periodic table: Periodic Table Group Valence Electrons; Group 1 (I) – Alkali metals: 1: Group 2 (II) – Alkaline earth metals: 2: Group 13 (III) – Boron group: 3:
RDR2 Online - Fishing At O'Creagh's Run. O’Creagh’s Run is a stunning alpine lake high in the Grizzlies East region of Ambarino. This beautiful lake has a cornucopia of exceptional fish to be caught. . Sockeye Salmon: Succulent fish meat, sales price $2.50, can hold 10 or 20 with the Trader role Materials Satchel Upgrade; .
valence electrons chart|how to calculate valence electrons